Have you ever wondered what makes aluminum such a versatile and widely used metal? The secret lies deep inside its atoms—in something called electron configuration.
Understanding aluminum’s electron configuration can unlock the reasons behind its unique properties and how it interacts with other elements. If you want to grasp what truly sets aluminum apart and why it behaves the way it does, this guide is made just for you.
Keep reading, and you’ll discover the simple science that explains a metal shaping our everyday lives.
Basic Electron Configuration
The basic electron configuration shows how electrons are arranged in an atom. It helps us understand the atom's behavior and properties. Each element has a unique pattern of electrons around its nucleus.
Electrons fill energy levels or shells. Inside these shells are smaller parts called subshells. Knowing this arrangement is key to grasping how aluminum atoms work.
Atomic Number And Electrons
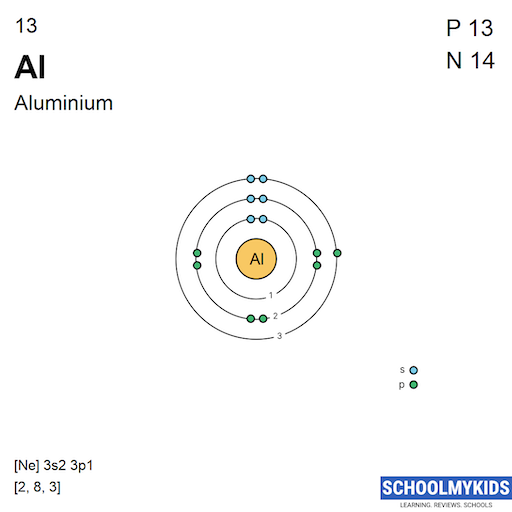
Aluminum has an atomic number of 13. This means it has 13 protons in its nucleus. It also has 13 electrons moving around the nucleus.
The number of electrons equals the atomic number in a neutral atom. These electrons are arranged in different shells to stay stable.
Electron Shells And Subshells
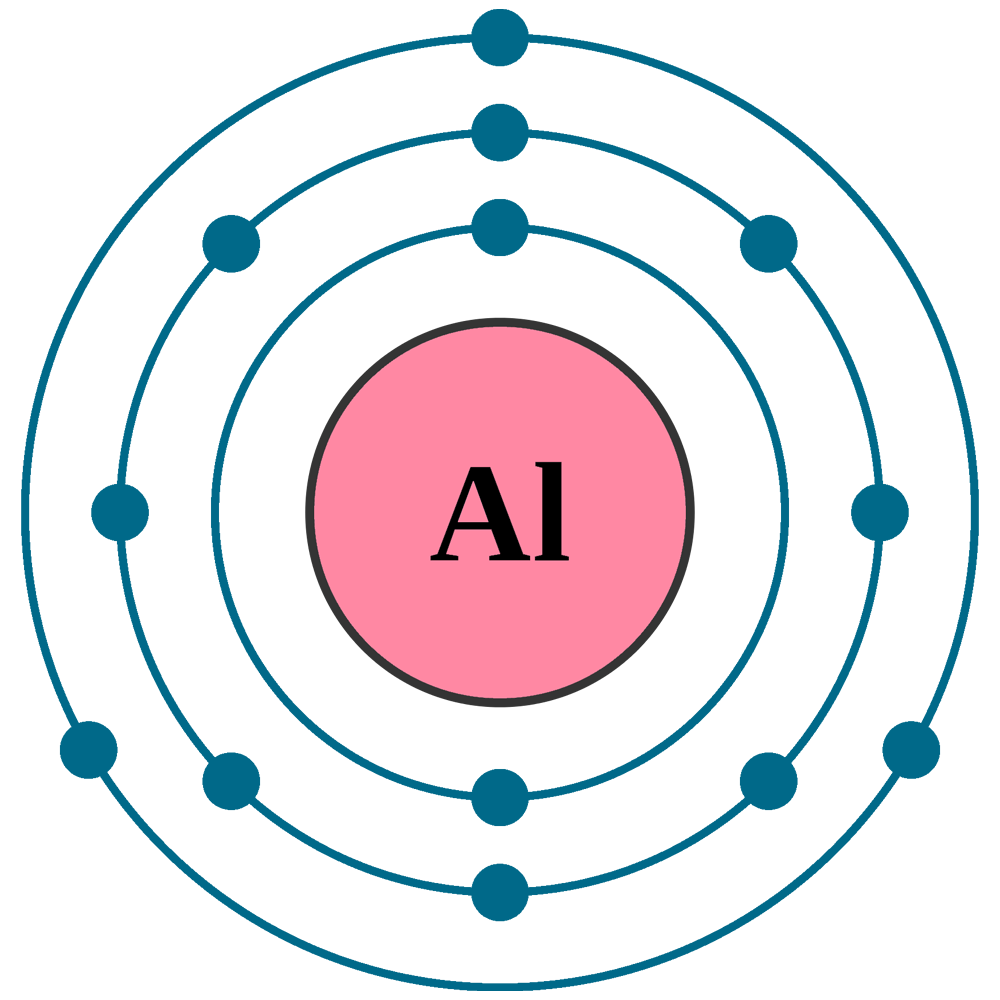
Electrons fill shells in order of energy levels. The first shell holds up to 2 electrons. The second shell can hold up to 8 electrons.
The third shell starts filling next. It holds the remaining 3 electrons in aluminum. These electrons occupy subshells named s and p.
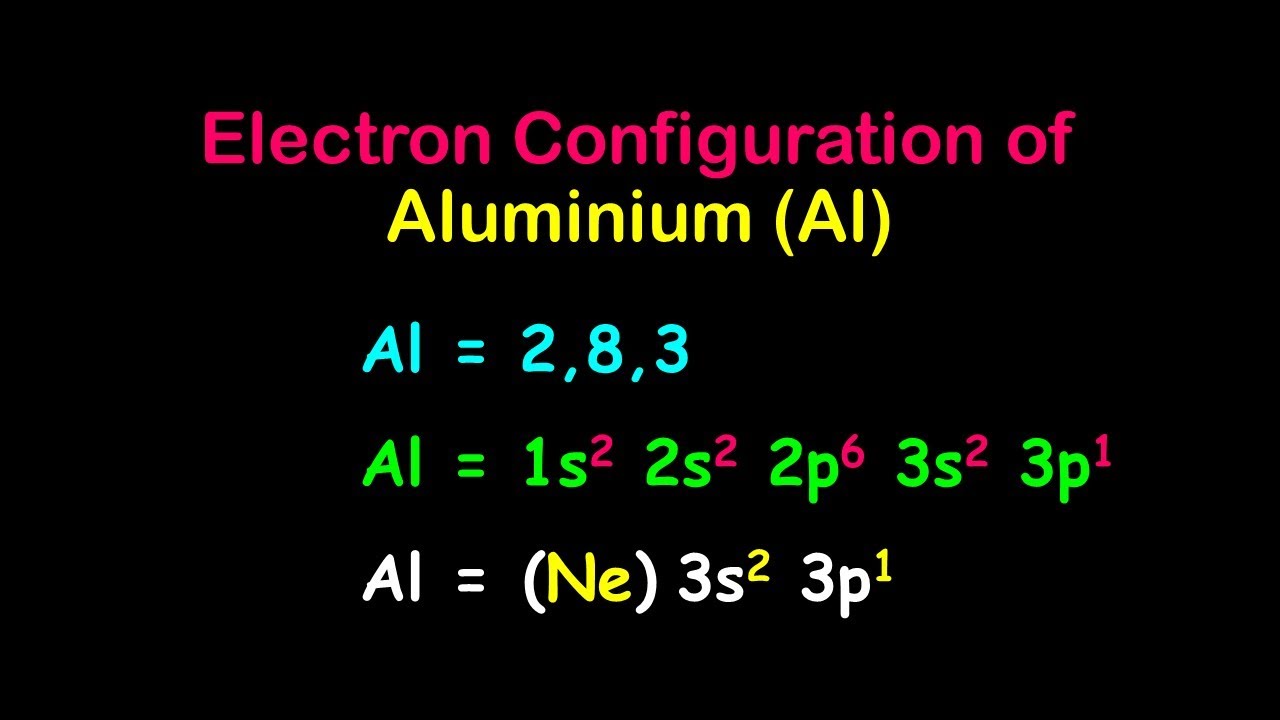
The electron configuration for aluminum is 1s² 2s² 2p⁶ 3s² 3p¹. This shows how electrons fill each subshell in sequence.
Aluminum's Electron Arrangement
Aluminum's electron arrangement reveals how its electrons fill energy levels. This arrangement helps explain aluminum's chemical behavior and properties. Each electron occupies a specific orbital, following strict rules. Understanding this setup is key to grasping aluminum's role in chemistry.
Filling Order Of Orbitals
Electrons fill orbitals in order of increasing energy. The rule is to fill the lowest energy orbitals first. For aluminum, electrons start in the 1s orbital. Then they move to 2s, 2p, 3s, and finally 3p orbitals. This order follows the Aufbau principle, which guides electron placement.
Ground State Configuration
Aluminum has 13 electrons in total. Its ground state configuration is 1s² 2s² 2p⁶ 3s² 3p¹. This means the first two electrons fill the 1s orbital. Next, two fill 2s and six fill 2p orbitals. The last three electrons occupy 3s and 3p orbitals. This setup gives aluminum its unique chemical traits.
Electron Configuration Notation
Electron configuration notation shows how electrons fill the shells and orbitals of an atom. It helps us understand the arrangement of electrons in an element. This arrangement affects the element’s chemical behavior and properties. For aluminum, knowing the electron configuration is key to grasping its reactivity and bonding patterns.
Full Notation
The full notation lists all the orbitals and the number of electrons in each. For aluminum, the full electron configuration is 1s² 2s² 2p⁶ 3s² 3p¹. This means aluminum has two electrons in the first shell’s s orbital, eight in the second shell’s s and p orbitals, and three in the third shell’s s and p orbitals. This detailed form shows the exact order in which orbitals fill.
Noble Gas Shorthand
The noble gas shorthand simplifies the full notation. It uses the symbol of the nearest noble gas before the element. For aluminum, the shorthand is [Ne] 3s² 3p¹. Here, [Ne] represents the electron configuration of neon, which covers the first ten electrons. This method makes writing and reading electron configurations faster and easier.
Credit: ajwaworld.com
Valence Electrons In Aluminum
Aluminum has three valence electrons. These electrons are in the outermost shell. They determine how aluminum reacts with other elements. Understanding valence electrons helps explain aluminum’s chemical behavior. It also shows why aluminum forms certain types of bonds.
Role In Chemical Bonding
Valence electrons in aluminum participate in bonding by sharing or losing electrons. Aluminum often loses its three valence electrons. This loss creates a positive ion with a charge of +3. This ion can bond with negative ions to form ionic compounds. Aluminum can also share electrons to make covalent bonds. These bonding types affect aluminum’s properties and uses.
Reactivity Implications
The three valence electrons make aluminum quite reactive. It easily loses electrons to achieve a stable state. Aluminum reacts with oxygen quickly, forming a protective oxide layer. This layer prevents further corrosion. The number of valence electrons also affects aluminum’s reaction with acids and bases. Understanding this helps in predicting aluminum’s chemical reactions in different environments.
Electron Configuration And Properties
Aluminum's electron configuration shapes many of its unique properties. Understanding this arrangement helps explain why aluminum behaves the way it does in different situations.
The electron configuration of aluminum is 1s² 2s² 2p⁶ 3s² 3p¹. This means aluminum has three electrons in its outer shell. These electrons play a key role in its chemical and physical features.
Conductivity And Metallic Character
Aluminum conducts electricity well. The outer electrons can move freely, allowing electric current to pass. This makes aluminum a good metal for wiring and electronics.
It also has a shiny, metallic look. Its electrons reflect light, giving aluminum a bright surface. The metal is lightweight but strong, useful in many industries.
Oxidation States
Aluminum commonly shows a +3 oxidation state. It loses three outer electrons to form positive ions. This helps aluminum bond with other elements easily.
Less common oxidation states are rare but can appear in special compounds. The +3 state dominates in most aluminum reactions, affecting its chemical behavior.
Credit: www.youtube.com
Excited States Of Aluminum
The excited states of aluminum occur when its electrons absorb energy and move to higher energy levels. This change alters the usual electron configuration, creating temporary states that differ from the ground state. These excited states play a crucial role in understanding aluminum's chemical and physical properties.
Electrons do not stay in excited states for long. They quickly return to their original positions, releasing energy in the process. This behavior is key to many applications in science and technology.
Electron Transitions
Electron transitions happen when an electron jumps between energy levels. In aluminum, an electron can move from the 3s or 3p orbitals to a higher orbital like 4s or 3d. These jumps require energy input, often from heat or light.
When electrons fall back to lower levels, they emit energy as light or heat. The energy released matches the difference between the two energy levels. This process defines the unique spectral lines of aluminum.
Spectroscopic Significance
Excited states in aluminum create distinct spectral lines. Scientists use these lines to identify aluminum in different materials. Spectroscopy helps measure aluminum's presence in stars, metals, and alloys.
The patterns of light from excited electrons offer clues about aluminum's environment. Changes in these patterns can show temperature, pressure, or chemical changes. This makes aluminum's excited states important for research and industry.
Credit: www.schoolmykids.com
Frequently Asked Questions
What Is The Electron Configuration Of Aluminum?
Aluminum’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p¹. This shows how its 13 electrons are arranged in shells and subshells.
Why Is Aluminum’s Electron Configuration Important?
Understanding aluminum’s electron configuration explains its chemical behavior. It helps predict bonding, reactivity, and placement in the periodic table.
How Does Aluminum’s Electron Configuration Affect Its Properties?
The 3p¹ electron makes aluminum reactive and a good conductor. Its configuration influences its lightweight, malleability, and corrosion resistance.
What Is The Valence Electron Configuration Of Aluminum?
Aluminum has three valence electrons in the 3s² 3p¹ orbitals. These electrons determine its bonding and chemical reactions.
Conclusion
Aluminum’s electron configuration helps explain its chemical behavior. It has 13 electrons arranged in specific shells. This setup shows why aluminum bonds easily with other elements. Understanding this helps in studies of chemistry and materials science. Keep these basics in mind to grasp aluminum’s role in nature and industry.
Simple yet important facts like these build a strong foundation. Exploring electron patterns opens doors to learning more about elements. It’s a small step toward deeper knowledge of the world around us.



0 Comments